CONTEC Sleep Apnea Meter, SpO2 Heart Rate Nose Air flow monitor Alarm+SW RS01
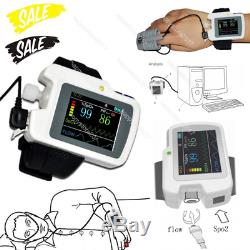
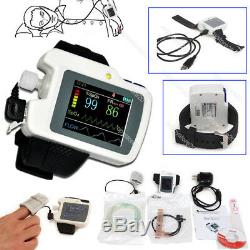

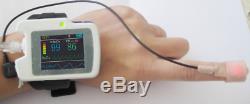


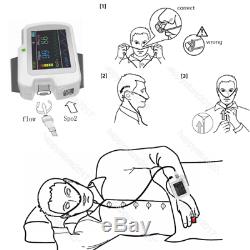
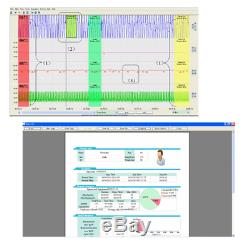
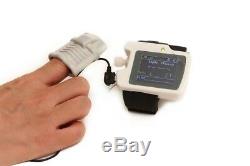


Introduction The RS01 device is suitable for the one who suffers with such diseases as SAHS (sleep apnea-hypopnea syndrome), Its functions including patients sleep apnea monitoring and SpO2 monitoring, which can be applied in hospital or at home. Features 1SpO2 and PR value display, pulse sound and bar graph indication. 2Pulse waveform and nose flow waveform display 3Alarms for low power, finger out and exceeding limits 4Battery status indication 5Real-time clock 6Timing power on/off 7Storage of multiple cases 8Continuous 12 hours data record 9Uploading data via USB port 10PC analysis software Performance Nose air flow measurement range: 0 rpm40 rpm, accuracy: ±2 rpm. SpO2 measurement range: 0%100% (resolution 1%), accuracy: 70%100%, ±2; less than 70%, unspecified.
PR measurement range: 30 bpm250 bpm, accuracy: ±2 or ±2% (whichever is greater). Resistance to surrounding light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%.
Power supply: 3.7 V rechargeable lithium battery. Safety type: internally powered equipment, type BF applied part. Accessories A power adapter A USB data line A Nasal cannula Two kinds of SpO2 probes A CD (PC software) A User Manual Physical characteristic Dimension: 69 mm (L)×50mm (W) ×17.3 mm (H) Weight: about 100g Working environment: Temperature: 10 40 Relative humidity: 75 % Atmospheric pressure: 700 hPa1060 hPa Storage environment: Temperature: -40 +55 Relative humidity: 95 % Atmospheric pressure: 500 hPa1060 hPa. EMS, UPS, TNT and DHL could be used for special requirement if you agree to pay more.
The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by. FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved.
The item "CONTEC Sleep Apnea Meter, SpO2 Heart Rate Nose Air flow monitor Alarm+SW RS01" is in sale since Wednesday, February 7, 2018. This item is in the category "Business, Office & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Medical & Lab Pumps". The seller is "doncurr-0" and is located in Beijing. This item can be shipped worldwide.- Unit Quantity: 5
- MPN: Does Not Apply
- Brand: contec
- Bundle Listing: Yes
- Type: RS01